Unless there's an acidic ingredient like lemon in baked goods, there won't be anything to neutralize baking soda(NaHCO3) to produce the needed carbon dioxide. So MAGIC also has an acidulant, in this case monocalcium phosphate (Ca(H2PO4)2), which has more aliases than a rap star--the IUPAC name being the best: calcium dihydrogenphosphate.
But why provide more acidulant than baking soda?  The dihydrogen phosphate ion (H2PO4-) ion reacts with baking soda's hydrogen carbonate ion(HCO3-) in a one to one ratio.
The dihydrogen phosphate ion (H2PO4-) ion reacts with baking soda's hydrogen carbonate ion(HCO3-) in a one to one ratio.
H2PO4-+ HCO3---> HPO42-+ H2O + CO2
Dissociating to provide two moles of H2PO4- , a mole of Ca(H2PO4)2 reacts with a pair of baking soda moles. But doubling the latter's molar mass of 84 still amounts to less than the acidulant's molar mass of 234 grams per mole, so for every gram of baking soda, you need more Ca(H2PO4)2, 1.39 to be exact. Finally, the primary ingredient is corn starch, which may also have the role of acting as a filler to accommodate teaspoon measurements but is definitely there to absorb moisture. Otherwise there would some reaction in the packaging stage or on the shelves, leading to a premature release of carbon dioxide.
Another surprise is the presence of calcium chloride(CaCl2) in most canned tomatoes. People from the Food Network believe the additive leads to lumpier sauces, and they advise cooks not to use tomatoes from cans. But one of the 3 brands in our pantry, Italpasta, is free of calcium chloride. What's more interesting is the strong industrial link between the ubiquitous calcium chloride and baking soda and soda ash(Na2CO3), a connection that is however vanishing, thanks to the less costly means of producing soda ash directly from the mineral trona
(Na3CO3HCO3.2H2O).
Exactly 150 years ago, in 1867, using a method he had developed a few years earlier, the Belgian chemist Ernest Solway founded a company in order to produce sodium carbonate(soda ash).
The compound is used mostly in glass-making to lower the melting point of silica, but it finds its way into many other consumer products. The method, which only consumes table salt and limestone, is brilliant in that it creates little waste. It reuses two intermediate products, carbon dioxide(CO2) and ammonia(NH3), and creates not only soda ash but our firming agent for tomatoes.
The overall reaction, as is often the case in both natural and industrial processes, is very deceiving:
The ammonium chloride meanwhile reacts with limewater(Ca(OH)2), releasing ammonia gas that is kept to re-initiate the cycle. The alkaline solution is produced by cooking calcium carbonate, which releases lime and which creates more carbon dioxide for the in-between reaction. Our calcium chloride is the byproduct of the step that releases ammonia.
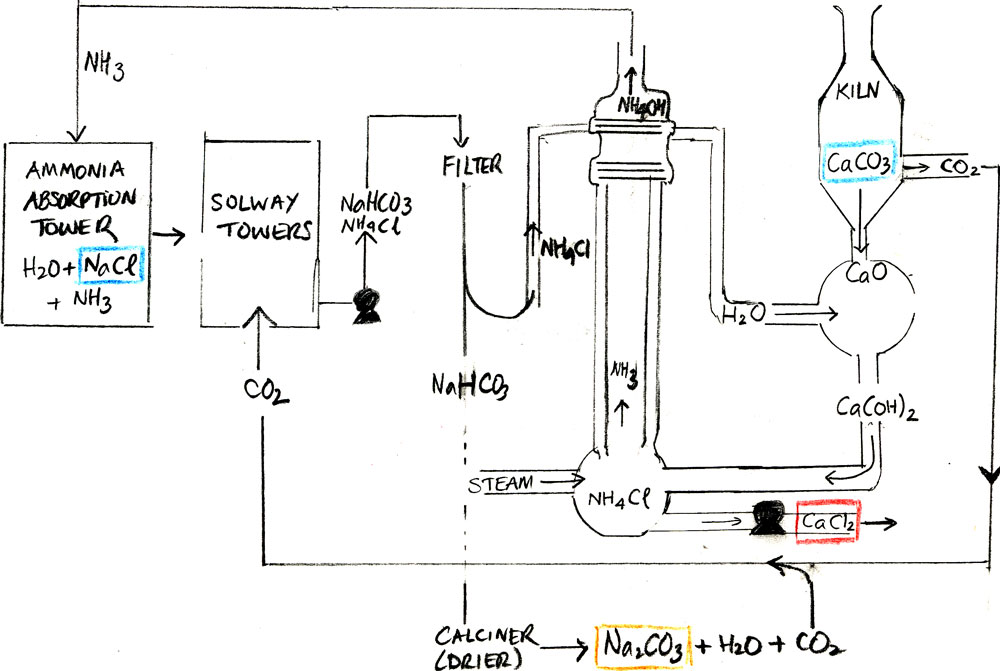
The water is slowly evaporated which forces out sodium chloride solid, leaving behind the more soluble calcium chloride.
It's because CaCl2 is hygroscopic--it easily attracts and holds on to water.
Compared to table salt, calcium chloride tastes much saltier, but it cannot be used as a substitute. Ca2+ plays an important role in cell signalling, and cells are sensitive to high levels of the ion. Not surprisingly the lethal dose that will kill 50% of mice is only 1940 mg CaCl2 /kg of body weight as opposed to 4000 mg/kg for table salt. But the concentrations of calcium chloride in canned tomatoes is nowhere near toxic levels. It's not only approved in the United States but in Europe, which is usually fussier over fuzzier matters.